The relationship between the microbiome and mitochondria is a fascinating and complex area of study, reflecting complex interconnections that have a profound impact on various aspects of human and companion animal health, influencing metabolic processes and disorders, immune function, neurodegenerative diseases, and aging.
This article will briefly explore the roles of the gut microbiome and mitochondria, their interactions, and their combined effects on human and companion animal health.
The microbiome
The microbiome itself is the complete set of microorganisms (microbiota), their genes, and the metabolites they produce in the microenvironment (habitat) in which they reside in and on an organism's body (e.g., the intestinal tract, mouth, skin).
The gut microbiome per se encompasses a diverse community of bacteria, viruses, fungi, and other microorganisms that inhabit the gastrointestinal tract. These microbes play essential roles in various bodily functions, including digestion and nutrient absorption, immune system regulation, and the production of vital metabolites.
Digestion and nutrient absorption
Certain gut bacteria produce enzymes that digest complex carbohydrates, such as dietary fiber. This microbial fermentation process produces short-chain fatty acids (SCFAs), like butyrate, propionate, and acetate, which provide energy to colon cells, have anti-inflammatory effects, and help the regulation of glucose and lipid metabolism.
Immune system regulation
The gut microbiome plays a crucial role in the development and modulation of the immune system. It helps in the education of immune cells and the maintenance of immune tolerance, preventing autoimmune diseases.
Metabolite production
Gut bacteria produce a number of metabolites which have specific roles, e.g., serotonin and gamma-aminobutyric acid (GABA), which can influence mood and cognitive functions via the gut-brain axis.
Mitochondria
The mitochondria (Fig. 1), often referred to as the powerhouse of the cell, are double-membrane organelles responsible for producing ATP through oxidative phosphorylation (energy production), involving the electron transport chain and ATP synthase. The energy thus produced is essential for various cellular functions: apoptosis, calcium signaling, the regulation of metabolic pathways, and overall cellular health.
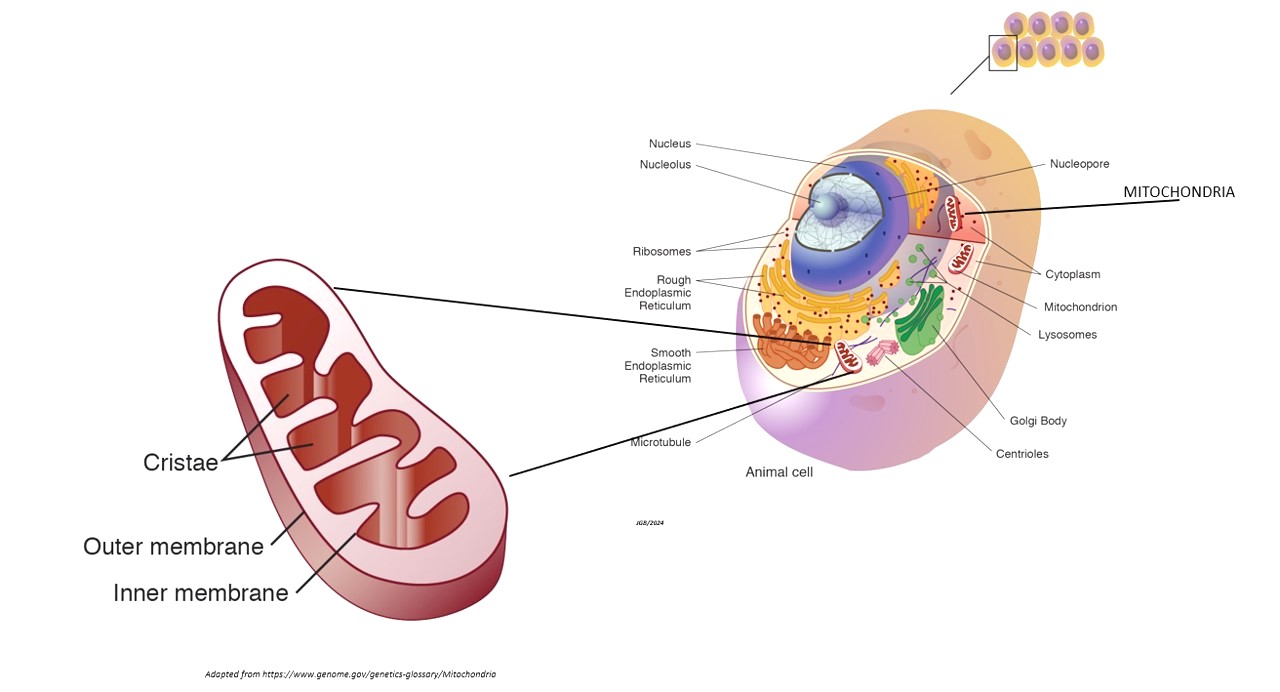
Apoptosis
Mitochondria play a pivotal role in apoptosis, a programmed cell death mechanism crucial for maintaining cellular homeostasis and preventing cancer.
Calcium signaling
Mitochondria help regulate intracellular calcium levels, which are vital for muscle contraction, neurotransmission, and other cellular processes.
Metabolic pathways
Mitochondria are central to the metabolism of carbohydrates, fats, and proteins, converting these macronutrients into usable energy.
Redox balance
Mitochondria produce Reactive Oxygen Species (ROS) as by-products of oxidative phosphorylation. While ROS play roles in cell signaling, excessive ROS can cause oxidative stress, damaging cellular components.
Antioxidant defense
Mitochondria possess antioxidant systems, such as superoxide dismutase (SOD) and glutathione, to neutralize ROS and maintain redox balance.
Interconnections between the gut microbiome and mitochondria
Recent research is beginning to reveal intricate connections between the gut microbiome and mitochondria, highlighting their collective impact on health (Fig. 2).
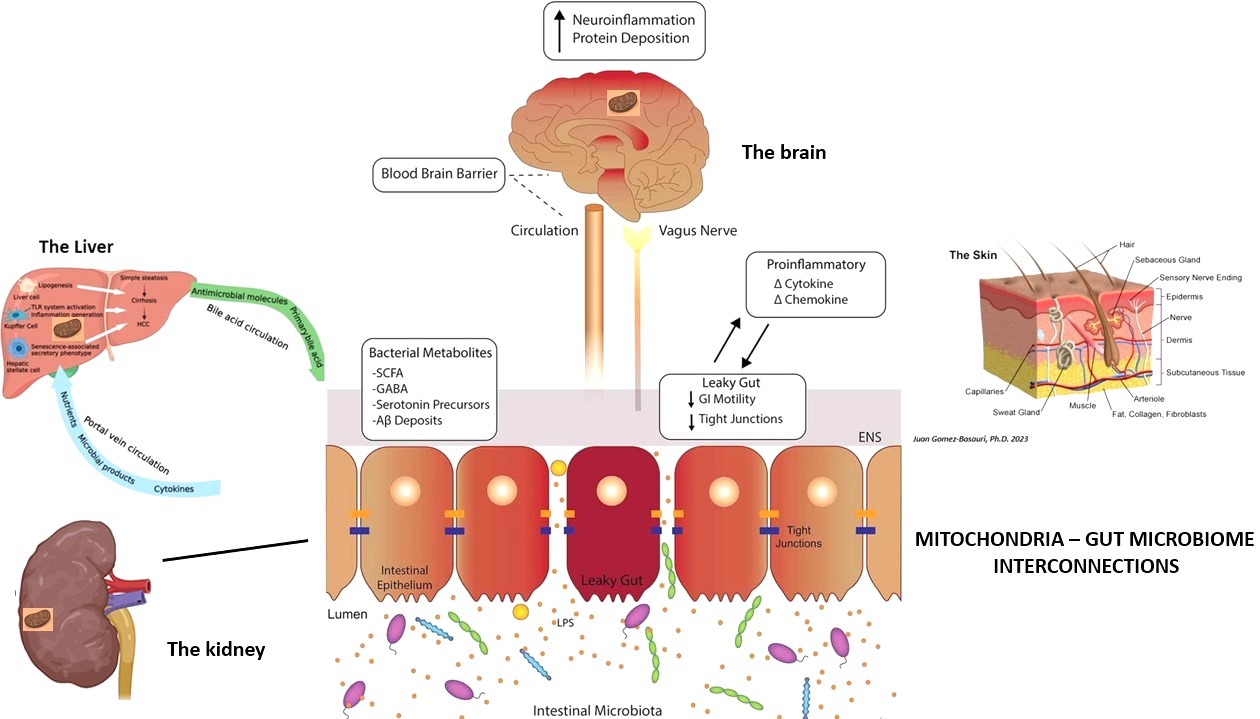
Microbial metabolites and mitochondrial function
SCFAs produced by gut bacteria can be used by mitochondria for ATP production. Butyrate, in particular, serves as an energy source for colonocytes and enhances mitochondrial function.
SCFAs can activate signaling pathways that promote mitochondrial biogenesis, enhancing mitochondrial function and energy production.
Inflammation and oxidative stress
Dysbiosis, or imbalance in the gut microbiome, can lead to chronic inflammation, contributing to mitochondrial dysfunction. Inflammatory cytokines can disrupt mitochondrial function, increasing ROS production and oxidative stress.
Elevated oxidative stress due to mitochondrial dysfunction can affect the gut barrier integrity, leading to increased intestinal permeability or 'leaky gut' and systemic inflammation, further exacerbating mitochondrial damage.
Gut-brain-mitochondria axis
Gut bacteria produce neurotransmitters and other signaling molecules that influence brain function. Mitochondria in neurons are crucial for neurotransmitter release and neuronal activity.
Dysbiosis and mitochondrial dysfunction are implicated in neurodegenerative diseases, such as Alzheimer's and Parkinson's disease. The gut-brain-mitochondria axis highlights the interdependence of these systems in maintaining neurological health.
Gut-liver mitochondria axis
Metabolites produced by gut microbiota consisting of short chain fatty acids and bile acids contribute to the regulation of hepatic homeostasis by interacting with mitochondria. The function and dynamics of the liver mitochondria, i.e., metabolism, biogenesis, and redox homeostasis, can be modulated by the gut microbiota.
Gut-kidney mitochondria axis
Mitochondria injury is the common damaged subcellular organelle in renal, intestinal and cardiovascular dysfunction. Mitochondrial structural and functional abnormalities, including impaired mitochondrial biogenesis, and oxidative stress contribute to the development and progression of cardio renal syndrome.
Combined impact on health and disease
The interplay between the gut microbiome and mitochondria has profound implications for health and disease. This relationship can influence metabolic disorders, immune function, neurodegenerative diseases, and aging.
Metabolic disorders
Dysbiosis can lead to altered energy metabolism, promoting obesity and insulin resistance. Mitochondrial dysfunction further exacerbates these conditions by impairing ATP production and increasing oxidative stress.
Probiotics, prebiotics, and dietary interventions targeting the gut microbiome can improve mitochondrial function and metabolic health. For example, increasing dietary fiber intake enhances SCFA production, supporting mitochondrial health.
Immune function
Dysbiosis and mitochondrial dysfunction can contribute to the development of autoimmune diseases. Restoring gut microbiome balance and mitochondrial function can modulate immune responses and reduce disease severity.
A healthy gut microbiome enhances the immune system's ability to fight infections, indirectly supporting mitochondrial function by reducing the burden of chronic inflammation.
Neurodegenerative diseases
Dysbiosis and mitochondrial dysfunction are linked to the pathogenesis of neurodegenerative diseases. Modulating the gut microbiome and improving mitochondrial function offer potential therapeutic strategies.
Maintaining a healthy gut microbiome and an optimal mitochondrial function is crucial for cognitive health, particularly in aging populations.
Aging
Mitochondrial dysfunction and chronic inflammation (inflammaging) are hallmarks of aging. The gut microbiome influences these processes by modulating inflammatory responses and mitochondrial health.
Strategies to enhance gut microbiome diversity and mitochondrial function, such as dietary interventions and probiotics, have the potential to promote healthy aging and extend lifespan.
Final thoughts
The gut microbiome and mitochondria are intricately linked, playing crucial roles in maintaining health and preventing disease. Their interactions influence metabolic processes, immune function, neurodegenerative diseases, and aging.
Understanding these relationships opens new avenues for therapeutic interventions, targeting both the gut microbiome and mitochondrial function. By harnessing the power of nutrition, functional bioactives, and lifestyle modifications, we can optimize these systems to improve overall health and longevity for both humans and companion animals.
By: Juan Gómez Basauri
Source: All Pet Food Magazine
You could be interested: A to Z of Pet Food: Oils & Omegas
About author
Juan Gómez Basauri, Ph.D.Doctor Juan Gómez Basauri is the founder and president of Magellan LLC, a company dedicated to developing new products, commercializing scientifically proven ingredients, and providing expert consulting services to the food and agriculture industries. He has more than 25 years of experience in leading positions and in charge of many business units in multinational companies, such as Ralston Purina and Alltech. Dr Gómez Basauri has a Bachelor of Science and Engineering from Universidad Federico Villareal in Lima, Perú. In addition, he has a Food Science MSc from the University of Leeds, England, and a Food Science Ph.D. from Cornell University, focused on Nutrition and Biochemistry. He was a fellow of the British Council and the Fulbright program, among other accomplishments. Dr Gómez Basauri is a sought-after speaker in the industry, and he has also published in trade journals and scientific publications.
Publisher Contents
Other microingredients
20/05/2025

































