A final draft of the updated model regulations for pet food and specialty pet food was voted on by the executive committee and will be presented to the full membership on July 31st for a vote.
There are numerous changes to the regulations that will impact the labeling for all pet food, pet treats and specialty pet food and treats. Below is a discussion of some of the main changes that will impact your pet food label design. Please note that small and very small packages with a total printable area of 40 or 12 square inches or less respectively will have different layout requirements due to space constraints
Changes to the front of the pack include the requirement for a verbatim intended use statement. This statement must be in the bottom ⅓ of the principal display panel and parallel to the bottom of the panel (front panel for packages that sit upright on the shelf and also on the bottom butt panel for packages that lay flat on the shelf), must be at least as large as the net quantity statement per FDA 16 CFR 500.21, must be in the same color and style font and on the same background color as the statement of net quantity and must be separated from the statement of net quantity and any other label material by the height of the 'N' above and below and by twice the width of the 'N' on either side.
{{editor}}
Some examples of the verbatim intended use statements include:
- 'Complete Dog Food' for foods that are complete and balanced for all life stages, including growth of large size dogs.
- 'Complete Puppy Food' or 'Complete Food for Puppies' for growth products that include growth of large size dogs.
- 'Complete Food for Adult Cats' for adult maintenance cat foods.
- 'Complete Food for Puppies (<70 lb. as an adult)' for growth diets that are not appropriate for the growth of large size dogs.
- 'Complete Food for Dogs (except puppies >70 lb. as an adult)' for all life stages products that exclude the growth of large size dogs.
Another major change is the replacement of the Guaranteed Analysis with a Pet Nutrition Facts section. This change was made to more closely align the nutritional information with the format on human food labels in order to help the consumer better understand what they are buying. The Pet Nutrition Facts is required to be included on all pet foods in a prominent place on the label (but not necessarily on the principal display panel). On most packages (except very small labels), this will be in a box set off by hairlines and will be all black or one-color type, printed on white or neutral background.
The heading 'Pet Nutrition Facts' will be centered in the top row of the box and must be twice the size of the other text in the box. The familiar household unit (cup, can, treat, etc) will be right justified in the next row and must include the weight in grams. A bold line must separate this information from the next row. Calorie content will appear in the next line of the box and will no longer be stated as kcal/kg. It must be stated in kcal/'familiar household unit' and will be left justified.
If the calories are determined by a feeding study, the word '(fed)' will appear after the value. Maybe provide an example of a few familiar household units. Underneath the calorie content, indented text will appear indicating the calories from protein, fat and carbohydrates. The Modified Atwater formula is still the correct way to determine the calorie content.
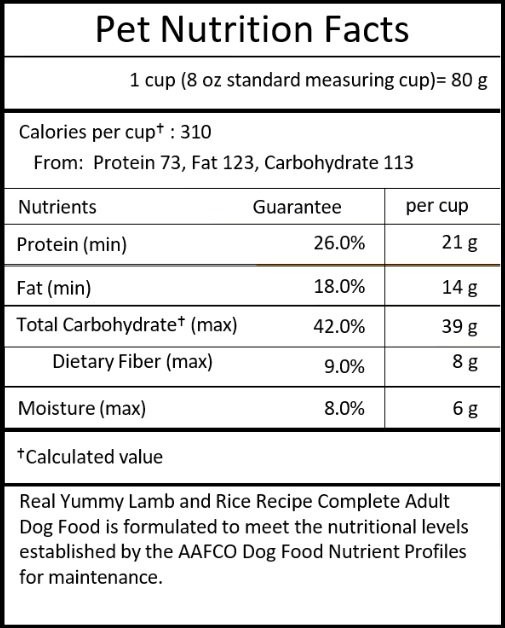
Following the calorie content will be the guaranteed nutrient levels. Protein, Fat, Carbohydrates (calculated value), Total Dietary Fiber and Moisture are the required guarantees for every label. These will be expressed in % and also in grams/'familiar household unit.' If you don't have dietary fiber data for your recipes, now would be a good time to begin testing to obtain these values. If you have additional nutritional guarantees, they may be included similarly to today's labels. Essential guarantees must still follow the order of the AAFCO nutrient profiles and nonessential nutrient guarantees are listed following the last essential guarantee.
The verbatim nutritional adequacy statement will still be required and must now live within the Pet Nutrition Facts box so that placement is consistent across all products. Feeding directions must be included on all labels and should be consistent with the intended use statement that is on the principal display panel.
There are some other minor changes that may impact your labeling, but the information above captures the most impactful of the proposed updates to the regulations. The implementation workgroup is proposing 6 years for the timeline for labeling in the market to comply with the new regulations. Consumer outreach and education has been a top priority and as more brands launch compliant labels, pet parents will be looking for this new format. This could potentially become part of the buying decision for savvy pet parents.
The North River Enterprises team is involved in several AAFCO workgroups helping to develop educational materials explaining the required changes once PFLM becomes a reality.
by Melissa Brookshire
You could be interested: White Label vs. Private Label: What’s the Difference—and Where’s the Opportunity?































